| GENE SLOVER'S US NAVY PAGES NAVAL ORDNANCE AND GUNNERY, VOLUME 1 CHAPTER 4 ARMOR AND PENETRATION |
| HOME INDEX CHAPTER 4 ARMOR AND PENETRATION A. TYPES OF ARMOR B. PENETRATION |
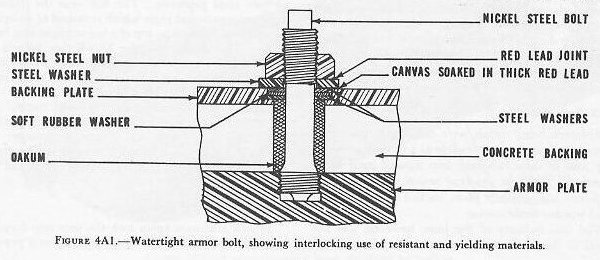
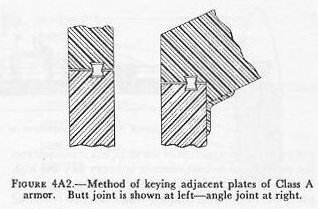
| A. Types of Armor 4A1. Early ship armor The idea of sheathing ships with projectile-resisting metal undoubtedly existed before any attempt was made to put it into practice. It is reported that a Korean admiral used ironclads in the late 16th century. The first European proposal to do so was made by Sir William Congreve in England in 1805, but the first ironmaster to make the attempt was John Stevens of Hoboken, N. J., some 7 years later. Stevens’ efforts did not meet with immediate success, but it does not appear that his approach to the subject was unscientific, for he had experimented until he knew the exact thickness of iron plate which would withstand the fire of any given gun. About 30 years after Stevens began the work, his son, Robert L. Stevens, felt that the work was far enough along to report the result to Congress. Yet another 12 years elapsed before Congress authorized the building of an armored floating battery. Meanwhile the French and the British laid the keels for a number of armored ships. In the bombardment of the Kinburn forts during the Crimean War, three French armored craft first demonstrated their usefulness. The widespread interest in armored ships was probably due to the fact that the rifling of the barrels of long-range guns was also occupying the attention of naval designers. The spin-stabilized bullet had been used in small arms for some time, and it was felt that a similarly designed long-range gun was feasible. Such a gun was developed, and by the last quarter of the 19th century, smooth-bore ordnance had disappeared from use in the navies of the civilized world. 4A2. Iron armor Although other metals were considered, only iron, wrought or cast, seemed feasible as a protective covering; and of these, wrought iron showed itself to be superior. The first practical armor consisted of 4 or 5-inch wrought-iron plate, backed by 36 inches of solid wooden timber. The iron industry of the time, however, was not equal to the production of the necessary heavy forgings. Therefore, even though early experiments had established solid plate as better than a built-up covering of laminated armor, naval designers were obliged to use plate built from thin layers, often inadequately bonded together. The armor of the Civil War ironclads was of this type. The problem before the armorers of the day was quite clear: to produce a very hard face plus a very tough back. However, methods of providing sound cementation between a hard faceplate and a thicker, heavier foundation were still to be discovered. 4A3. Steel armor Before any process for the production of such bonded armor was developed, the Bessemer converter, followed a few years later by the Siemans-Martin open-hearth process, changed the course of development. The French, in 1876, produced a 22-inch mild steel plate (which is said to have been hammered to that dimension from a thickness of 7 feet) that resisted the fire of all guns then in use. It was the best armor produced up to that time, but its tendency to crack led to a return to research in built-up armor. 4A4. Compound armor The next advance resulted from the development of two rival processes. The first was the Wilson-Cammel compound plate which consisted of an open-hearth steel face cast on top of a hot wrought-iron back plate. The second was the Ellis-Brown process of cementing a steel face to an iron back by pouring molten Bessemer steel between them. In both cases, the plates were rolled after compounding. Actually, only a small advance had been made. All the efforts of the steel men and Naval designers had amounted to a mere 25 percent advantage: 10 inches of compound armor was only about the equal of 12 1/2 inches of iron. It was some progress, but not much. 4A5. Nickel-steel armor French engineers again took the next step forward when, in 1889, Schneider introduced about 4 percent of nickel into his steel plate, which increased its strength and toughness. His plate was hammer-forged, annealed, tempered, oil-quenched, and then reannealed. This new process added another 5 percent of resistance to the 25 percent already gained by the makers of compound plate. This type of plate was used by the United States to protect the old battleships Texas, Maine, and Oregon. 4A6. Harvey armor (carburized nickel steel) The next important development was American and, oddly enough, also originated in New Jersey, not far from the Stevens plant where protective plating for naval craft had had its beginning about 80 years before. In 1890, H. A. Harvey, of Newark, invented a process which added about 15 percent more strength to the plate described above. The new method consisted of carburizing the face of a plate of nickel steel by holding it at about the temperature of molten cast iron for 2 or 3 weeks with the face in contact with bone charcoal. This increased the carbon content of the outer inch of the face from about one-fifth of 1 percent to slightly more than 1 percent. The entire plate was then quenched, first in oil and then in water, and the result was both a hardening of the face and a toughening of the back. Later, the water dipping was replaced by cooling with a dense, high-pressure water spray. It was soon found that this type of plate could be reforged at a low temperature after carburizing, reducing its thickness by from 10 to 15 percent without loss of strength. The resultant plate had the strength of iron armor half again as thick as itself. 4A7. Krupp armor (carburized nickel chrome alloy steel) The hardening effect of adding chromium to nickel steel had been discovered before the above development was completed, but the resultant alloy was too difficult for the industry to handle until the Germans discovered suitable methods. Krupp at that time used illuminating gas as a carburizing agent instead of bone charcoal, but the industry at a later date returned to the use of a solid carbonaceous material for this purpose. 4A8. Krupp armor (decrementally hardened) The important decremental hardening process was introduced by Krupp shortly after the development of carburizing. The Krupp armor was processed by burying the plate, all but the face to be hardened, in clay, and exposing the face to a high, quick heat. This heat traveled from the face of the plate toward the back in an evenly descending plane, and when the critical heat for hardening had penetrated to from 30 to 40 percent of the thickness, the plate was removed to a spray pit and chilled by water played at first on the face alone and, a few moments later, on both sides of the plate together. This decremental face-hardening, as it was called, is still the general process by which modern protective armor is produced, though further refinement of the method constantly goes forward. This process may be applied to carburized or noncarburized armor as a final treatment. 4A9. Class A armor The carburized face-hardened plating described in the foregoing article is known as Class A armor. Its use is protection of the vertical surfaces around the more vital parts of heavily armored ships-the sides, the turrets, the barbettes, etc. The impact of a projectile against such surfaces would necessarily be at a very small angle of obliquity (1) and as such would have to be withstood by a very hard face to resist the initial impact, plus great backing strength to absorb the shock. Class A armor must defeat a projectile by stopping it, by breaking it up, or by rupturing the explosive cavity (thus reducing its effectiveness even though it penetrates the plate). Such armor must be of considerable weight, and naval design admits of the use of only a limited quantity of it. Enclosing the hull of a ship with heavy armor not only does not add to the strength of the craft, but actually diminishes it, for the great mass, affixed to the framing members and other strong points, complicates the stresses. For that matter, all armor represents dead weight, and naval designers must balance the requirements of essential protection against dead weight. (1) The angle of obliquity is measured between the axis of the projectile and the normal to the plate at the point of impact. Class A armor can be machined only with difficulty, and cannot be fitted snugly against the skin of a ship. The accepted method is to suspend it from the strong points of the hull by means of extended watertight bolts (fig. 4A1) which allow about 2 inches clearance between the armor and the hull, and then to fill the space with concrete. Abutting edges are keyed together, and plates which meet at an angle are rabbeted (fig. 4A2). The chemical analysis of a typical modem plate is about as follows: Carbon_______________________0.33 percent Nickel________________________3.33 percent Chromium_____________________2.00 percent Manganese_____________________0.30 percent Silicon________________________0.07 percent Phosphorous___________________0.016 percent Sulphur_______________________0.02 percent Iron_________________________93.93 percent |
 |
 |
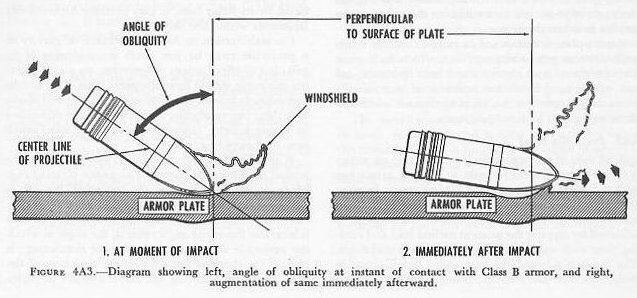
| 4A10. Class B armor Armor designed for the protection of horizontal surfaces, and otherwise, where the anticipated angle of obliquity is great, is physically quite different, although chemically about the same. Here, instead of boldly meeting force with resistance, advantage can be taken of the tendency of a projectile to ricochet. This glancing rebound is best achieved when the impact of the projectile is met with a plate that gives slightly, thus spreading the force over a wider area. Moreover, the curvature of the depression induced by the impact tends to pick up the curvature of the ogive, further inducing the projectile to rebound harmlessly away by increasing the angle of obliquity immediately after the instant of contact. (See fig. 4A3.) Homogeneous armor can be used in this application. Homogeneous armor is not face-hardened. In thicknesses 3 inches and less, it is called “STS” (Special Treatment Steel). In thicknesses greater than 3 inches, it is termed class B armor. It can be integrated with the structure of the ship, but it complicates the problem of weight, since it is heavy and lies high in the ship’s structure, thereby shifting materially what would otherwise be the ship’s normal center of gravity. 4A11. Miscellaneous armor Two minor classes of armor are cast and light. In the present state of its development, cast armor is not very satisfactory. It is used at points where plate-armor would be difficult to fit, such as turret range-finder end windows and sight hoods; attempts have been made to use it for boiler uptake grating plates. It has a future, because of the facility and speed with which castings can be manufactured, but metallurgical advances must take place before extensive use can be made of it. Light armor is a rough designation for any armor less than 2 inches thick. It is for the most part constructed like heavier armor, though some is compounded armor consisting of a hard face fused to a tough back. Its prospective usefulness as a protection for aircraft personnel and engines makes this one of the most important fields of ballistic research. Weight, however, is again a limiting factor. Nonferrous armor (of aluminum alloy) can be used to protect against fragments. And plastic armor may be used for personnel. |
 |
 |